44 fda guidance use of symbols on labels
Code of Federal Regulations Title 21 - Food and Drug Administration (2) The label of general purpose laboratory equipment, e.g., a beaker or a pipette, shall bear a statement adequately describing the product, its composition, and physical characteristics if necessary for its proper use. (e)(1) The labeling for analyte specific reagents (e.g., monoclonal antibodies, deoxyribonucleic acid (DNA) probes, viral ... › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims |FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ...
FDA issues additional procedural notice on consumer research on ... No Comments. The FDA is conducting the consumer research on a potential symbol, which is intended to be a stylized representation of the nutrient content claim "healthy," while at the same time developing a proposed rule that would update when manufacturers may use the "healthy" nutrient content claim on food packages.
Fda guidance use of symbols on labels
Carton and Container Labeling Resources | FDA Chapter <7> (Labeling) provides definitions and labeling standards for official USP articles including strength expression for injectable products, pharmacy bulk packages, imaging bulk packages, labeling for ferrules and cap overseals, aluminum use in parenteral nutrition, and expiration and beyond the use date. Labeling Policies | Food Safety and Inspection Service Guidance for Industry and FDA Staff: Whole Grain Label Statements (Feb 17, 2006; PDF Only) Draft guidance document issued by the Food and Drug Administration intended to provide guidance to industry about what the agency considers to be "whole grain" and to assist manufacturers in labeling their products. Labeling Guidances | FDA Labeling Guidances. CVM GFI #45 Guideline for Uniform Labeling of Drugs for Dairy and Beef Cattle. Guidance for Industry: Voluntary Labeling Indicating Whether Foods Have or Have Not Been Derived ...
Fda guidance use of symbols on labels. FDA Issues New Guidance on Updating Labeling for Generics After ... The FDA has released draft guidance for applicants and holders of an abbreviated new drug application (ANDA) on updating their labeling after revisions to the approved labeling of a reference listed drug (RLD) on which a generic drug is based. The draft revises guidance from April 2000 titled Revising ANDA Labeling Following Revision of the RLD Labeling and will replace it once finalized. Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 801.1 - Medical devices; name and place of business of manufacturer, packer or distributor. § 801.3 - Definitions. § 801.4 - Meaning of intended uses. § 801.5 - Medical devices; adequate directions for use. Fda Medical Device Label Symbols - fda launches new webpage to promote ... Here are a number of highest rated Fda Medical Device Label Symbols pictures upon internet. We identified it from trustworthy source. Its submitted by supervision in the best field. We agree to this nice of Fda Medical Device Label Symbols graphic could possibly be the most trending topic afterward we allowance it in google pro or facebook. Medical Device Labeling New ISO 15223-1 FDA Guidance Recommend UDI ... However, the guidance provides a recommendation that labelers consider using symbols that are internationally recognized via a standards development organization and that the use of such symbols is very much consistent with 21 CFR 801.15, Medical devices; prominence of required label statements; use of symbols in labeling.
Code of Federal Regulations Title 21 - Food and Drug Administration CFR - Code of Federal Regulations Title 21. The information on this page is current as of Mar 29, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 801.109 Prescription devices. A device which, because of any potentiality for harmful effect, or the method of its use, or the ... › medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA Manufacturers should look to the final rule, not the withdrawn 2004 guidance, when determining their use of symbols in new labeling and when making labeling updates. 14. FDA Final Rule on the Use of Symbols in Labeling - loring In an attempt to remedy this issue and harmonize the U.S. device labeling requirements with international regulatory requirements (e.g., ISO 15223-1:2021 and BS EN 980:2008), the FDA has issued a final rule that revises its "medical device (and certain biological product) labeling regulations to explicitly allow for the optional inclusion of graphical representations of information, or ... FDA Food Packaging Guidelines for 2022 - Newprint Jan 19, 2022 • Maja Mandic. These food packaging guidelines can help everybody who is involved in creating food packaging to make sure that the packaging complies with the regulations. When companies think about food packaging, it is usually focused on making it attractive and enticing the customers to buy the product.
Instructions for Use — Patient Labeling for Human Prescription Drug and ... This guidance provides recommendations for developing the content and format of a patient Instructions for Use (IFU) document for human prescription drug and biological products, as well as drug ... Final FDA Guidance on Safety Considerations for Medication Container ... On May 18, 2022, the FDA issued final guidance entitled "Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors." In this guidance, the FDA provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a product's container label and carton labeling are designed to promote safe dispensing, administration ... FDA Issues Final Guidance on Instructions for Use Documents This guidance finalizes the draft guidance that FDA issued in July 2019 titled "Instructions for Use — Patient Labeling for Human Prescription Drug and Biological Products and Drug-Device and Biologic-Device Combination Products — Content and Format, Guidance for Industry.". In the final guidance, FDA added clarifying language ... › food › guidance-regulation-food-andGuidance Documents & Regulatory Information by Topic (Food ... May 16, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ...
Use of Symbols in Labeling - FDA
Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 801.15 Medical devices; prominence of required label statements; use of symbols in labeling. (a) A word, statement, or other information required by or under authority of the act to appear on the label may lack that prominence and ...
Guidances | FDA Guidance documents describe FDA's interpretation of our policy on a regulatory issue (21 CFR 10.115 (b)). These documents usually discuss more specific products or issues that relate to the ...
United States: Final FDA Guidance On Safety Considerations For ... On May 18, 2022, the FDA issued final guidance entitled "Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors." In this guidance, the FDA provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a product's container label and carton labeling are designed to promote safe dispensing, administration, and ...
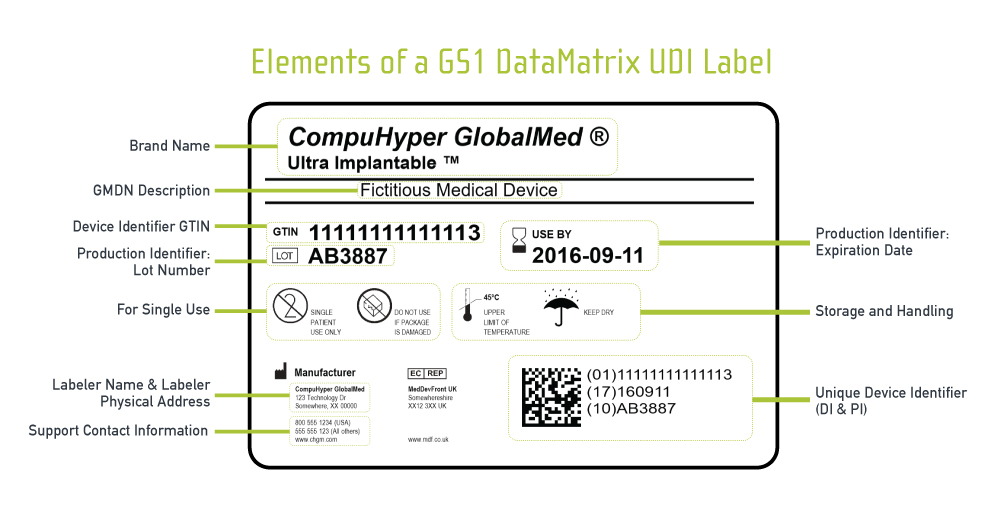
New FDA Guidance for UDI Symbol Use. In other words, the FDA says manufacturers should include the UDI symbol on labels that have more than one barcode or scannable graphic. While using the UDI symbol remains optional, practitioner advocacy groups encourage manufacturers to use it on labels with more than one barcode. Ultimately, using the UDI symbol ensures health care providers ...
Recognized Consensus Standards - Food and Drug Administration Recognized Consensus Standards. This document specifies symbols used to express information supplied for a medical device. This document is applicable to symbols used in a broad spectrum of medical devices, that are available globally and need to meet different regulatory requirements. These symbols can be used on the medical device itself, on ...
FDA Issues Final Rule on Symbols in Labeling - Getupeducation FDA back in June 2016 issued a final rule to allow for the use of standalone symbols on medical devices and in vitro diagnostic (IVD) labels to align with international standards. In addition to allowing the use of standalone symbols, the final rule also permits the use of the symbol statements "Rx only" and "Rx only" for prescription devices.
› regulatory-information › search-fdaCPG Sec.140.500 Metric Declarations of Quantity of Contents ... In support of this policy, the agency has developed the following guidance on the use of the metric system in declaring the net quantity of contents on the labels of FDA-regulated commodities.
FDA Provides Draft Guidance for Using ctDNA Biomarker in ... - FDAnews The FDA has released a draft guidance for cancer drug sponsors on the design of clinical trials that use circulating cell-free plasma-derived tumor DNA (ctDNA) as a biomarker. Because ctDNA can be measured in the bloodstream, the biomarker can be used in the early stages of a clinical trial, including to detect a targetable alteration, to determine specific study populations, to reflect ...
FDA Issues Final Rule on Symbols in Labeling FDA back in June 2016 issued a final rule to allow for the use of standalone symbols on medical device and in vitro diagnostic (IVD) labels in an effort to align with international standards. In addition to allowing the use of standalone symbols, the final rule also permits the use of the symbol statements "Rx only" and "Rx only" for ...




![FDA - Labeling - [PDF Document]](https://reader019.documents.pub/reader019/reader/2020031315/58a1ddb31a28abb6678b698b/r-1.jpg)



Post a Comment for "44 fda guidance use of symbols on labels"